National
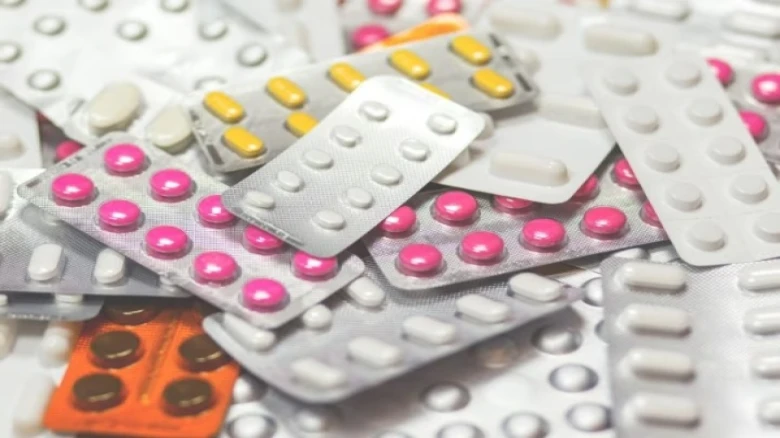
Among the prohibited medications were those used to treat common illnesses, coughs, and fevers...
Digital Desk: The government has prohibited 14 fixed-dose combination drugs, including Nimesulide and Paracetamol dispersible pills and Chlorpheniramine Maleate and Codeine syrup, claiming that these medications have "no therapeutic justification" and may pose a "risk" to consumers. On Friday, the Union Health Ministry issued a notification in this regard.
Nimesulide + Paracetamol dispersible tablets, Chlorpheniramine Maleate + Codeine Syrup, Pholcodine + Promethazine, Amoxicillin + Bromhexine and Bromhexine + Dextromethorphan + Ammonium Chloride + Menthol, Paracetamol + Bromhexine+ Phenylephrine + Chlorpheniramine + Guaiphenesin and Salbutamol + Bromhexine are enlisted among the banned drugs.
The decision was made in response to the suggestions of an expert committee. According to the expert committee, there is "no therapeutic justification" for this FDC (fixed dose combination), and the FDC may pose a risk to humans.
As a result, in the broader public interest, the manufacture, sale, or distribution of this FDC is prohibited under section 26 A of the Drugs and Cosmetics Act of 1940.
FDC medications are those that include a set ratio of two or more active pharmaceutical ingredients (APIs). In 2016, the government announced a ban on the manufacture, sale, and distribution of 344 drug combinations after an expert panel appointed by the Supreme Court concluded that they were being sold to patients without scientific evidence, and the order was challenged in court by the manufacturers.
Those 344 medication combinations include the 14 currently prohibited FDCs.
Leave A Comment