International
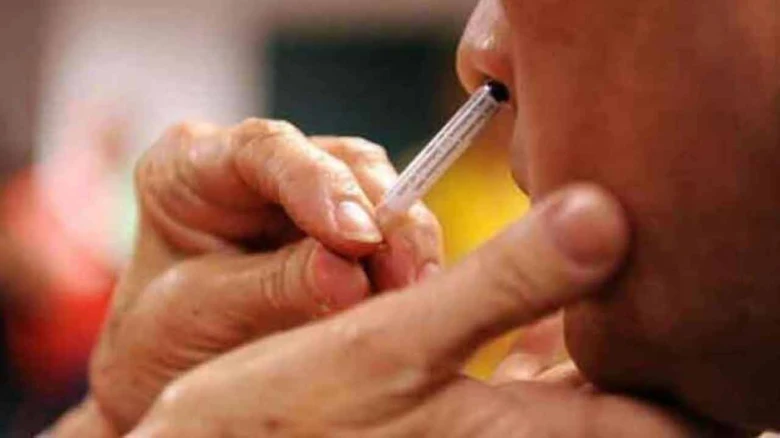
This is the world's first intranasal Covid vaccination to be approved for the primary 2-dose schedule as well as a heterologous booster dose...
Digital Desk: Bharat Biotech's nasal vaccine will cost Rs 800 plus taxes at private hospitals, and slots may now be booked through the CoWin portal, the pharmaceutical company announced on Tuesday (December 27).
Reportedly, the nasal vaccine, iNCOVACC, will be available in the fourth week of January.
For large-scale procurement by the central and state governments, iNCOVACC would be priced at Rs 325 per dosage.
iNCOVACC is being rolled out as a booster shot for those above the age of 18.
This is the world's first intranasal Covid vaccination to be approved for the primary 2-dose schedule as well as a heterologous booster dose, Bharat Biotech said.
Bharat Biotech said in a statement that the phase-III trials and heterologous trials were carried out at 14 and 9 sites across the country, respectively.
According to the pharma giant, those who received the vaccine showed significant levels of antibody levels in their saliva during the studies. Mucosal IgA antibodies in the upper respiratory tract may help reduce infections and infection transmission, according to the study.
Bharat Biotech gained authorization from the central drug regulator earlier this month to use the nasal vaccination as a heterologous booster dosage. With the heterologous booster system, a different vaccination than the one in the initial dose can be given to the patient.
Bharat Biotech stated that the nasal delivery system was developed to be cost-effective in low- and middle-income nations.
"We have achieved the goals we set for ourselves during this pandemic. We have developed COVAXIN and iNCOVACC, two COVID vaccines from two different platforms, with two different delivery systems. The vectored intranasal delivery platform gives us the capability for rapid product development, scale-up, easy and painless immunization during public health emergencies and pandemics," Dr. Krishna Ella, executive chairman of Bharat Biotech said in a statement. He expressed gratitude to the government for its support and direction, particularly the Health Ministry.
iNCOVACC was created in collaboration with Washington University in St. Louis, which designed and produced the recombinant adenoviral vectored construct and tested its efficiency in preclinical investigations, Bharat Biotech said.
The nasal vaccine dosages are stable between 2-8 degrees Celsius, allowing for convenient storage and distribution, according to the pharmaceutical company.
Leave A Comment