Digital Desk: The
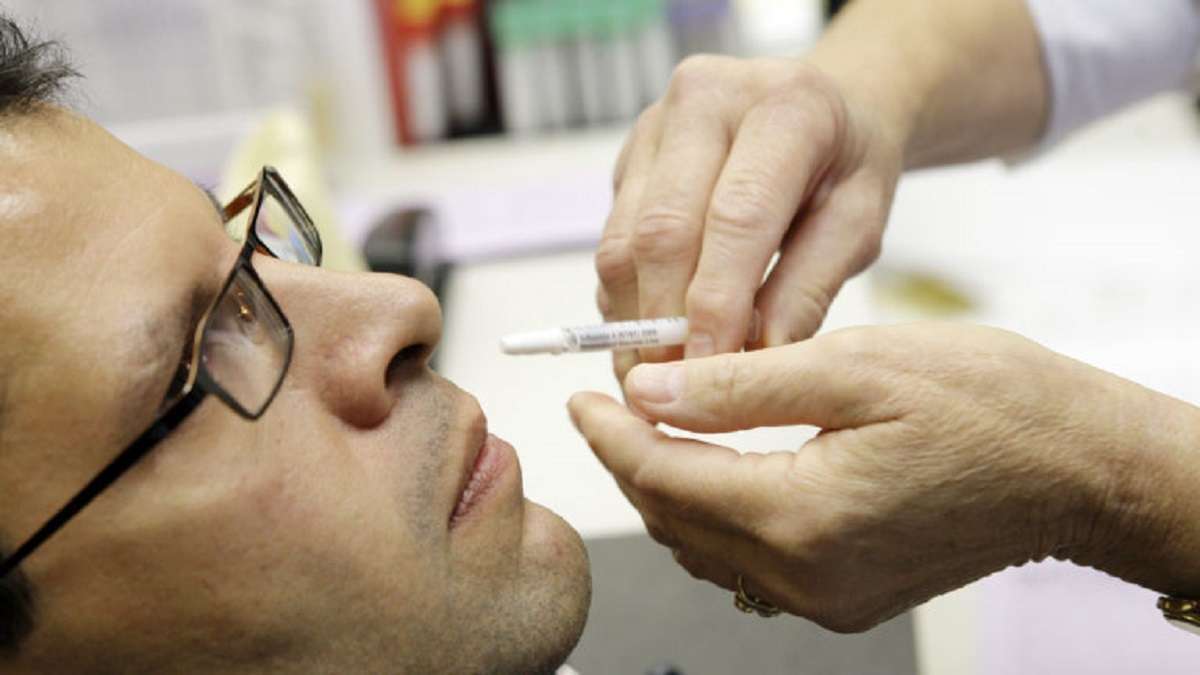
Drug Controller General of India (DCGI) recently approved Bharat Biotech, which is an Indian multinational biotechnology company headquartered in the city of Hyderabad, for Intra – Nasal Vaccine Phase -3 trials.
According to reports, an Intranasal vaccine would be simple to inject and also will reduce the use of needles and syringes, unlike before.
Also Read:
Cristiano Ronaldo illuminates Burj Khalifa on girlfriend Georgina Rodriguez’s birthday, spends 50,000 pounds
The approval for the clinical trials was granted on Friday and the trials would evaluate the nasal vaccine for both the two-dose primary schedule and also to use as a booster dose schedule.
According to Chairman of
Bharat Biotech Krishna Ella, it would also impact the overall cost of a vaccination drive. Notably, BBV154 is an intranasal replication-deficient chimpanzee adenovirus
SARS-CoV-2 vectored vaccine.
Leave A Comment