Sports
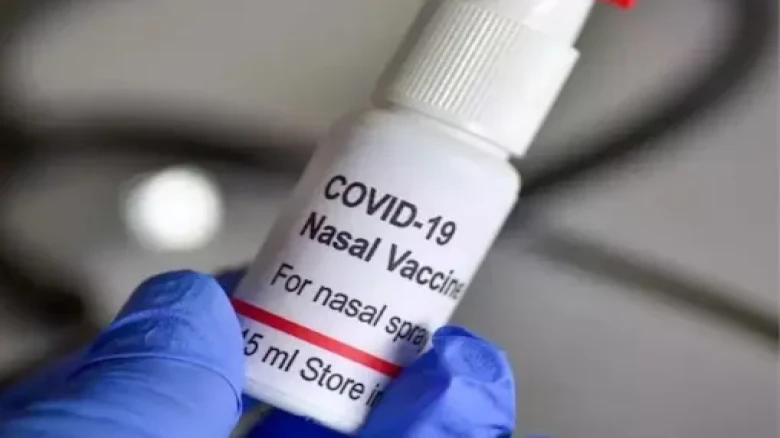
The nasal vaccine can be taken six months after the second dosage...
Digital Desk: The Drugs Controller General of India (DCGI) has approved vaccine maker Bharat Biotech's Intranasal 'Five Arms' Covid-19 booster dose for restricted use.
Reportedly, the emergency use authorization (EUA) has been given to the nasal vaccine iNCOVACC for restricted emergency use as the third dose for adults, irrespective of having been administered Covaxin or Covishield vaccine doses.
According to the vaccine manufacturer, the nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa. Thus, Intranasal immunization of ChAd-SARS-CoV-2-S can create an immune response in the nose, which is the virus's point of entry, thereby guarding against illness, infection, and transmission.
The nasal vaccine can be taken six months after the second dosage. It is simple to administer as it is non-invasive and needle-free.
Bharat Biotech stated that the intranasal vaccine generates a wide immune response. It is expected to prevent both infection and transmission of Covid-19.
Leave A Comment